The electronics inside a medical device carry a burden that consumer products do not. A failure in the field is not an inconvenience; it is a potential patient safety event. It triggers regulatory notifications and, in severe cases, recalls that can span thousands of units across multiple markets. This reality shapes every aspect of how medical device circuit boards are designed, built, and documented. It is why the industry operates under ISO 13485, a quality management system that governs the entire manufacturing lifecycle with a rigor far exceeding general industrial norms.
For companies developing new devices, the challenge isn’t simply achieving compliance. It’s achieving compliance without sacrificing the speed needed to meet clinical timelines and commercialization windows. A pervasive assumption holds that regulatory discipline and aggressive lead times are fundamentally in tension—that the rigor of Device History Records, the precision of labeling, and the overhead of process validation must inevitably slow production.
This assumption is false. It is a dangerous myth perpetuated by manufacturers who bolt compliance on as an afterthought rather than building it into the architecture of their operations. When a PCBA manufacturer operates under a mature ISO 13485 system, the very mechanisms that ensure traceability and repeatability become the enablers of speed. Real-time data capture eliminates retrospective paperwork. Validated processes remove the need for per-unit firefighting. Continuous audit readiness prevents the disruptive scrambles that derail schedules. Validated systems don’t slow you down. Ad-hoc compliance does.
To understand this, one must look past the ISO 13485 certificate on the wall and examine the systems that satisfy audits without stretching lead times.
What ISO 13485 Certification Means for PCB Assembly
ISO 13485 is a quality management system standard, not a product standard. The distinction is critical. When a PCBA manufacturer holds ISO 13485 certification, an accredited auditor has verified that the organization runs a documented, controlled, and monitored system for managing quality. The certification does not attest to the quality of any single product; it attests to the discipline of the system that produces those products.
While the standard shares an ancestor with the more common ISO 9001, ISO 13485 is far more prescriptive in areas impacting patient safety. Where ISO 9001 allows flexibility, ISO 13485 mandates specific controls for risk management, process validation, traceability, and post-market surveillance. For a contract manufacturer, this means the entire production environment—from workflows and equipment to record-keeping—operates at a level far beyond the norms of consumer electronics.
The presence of an ISO 13485 certificate is table stakes for medical device work. Its absence signals that a partner is not equipped to meet regulatory expectations. But the certificate alone doesn’t reveal operational maturity. It verifies that a system exists, not whether that system drives operational excellence or is merely maintained to pass an annual audit. The difference lies in the architecture of that system.
The Architecture of Traceability: Device History Records in PCBA
The Device History Record (DHR) is the complete documentation package proving what was built, how it was built, and by whom. For every medical device assembly, an auditor must be able to reconstruct its genealogy with forensic precision. This means knowing the specific lot of every component on the board, the batch identifier of the solder paste, the machine IDs for the pick-and-place and reflow oven, the operator who ran the line, the environmental conditions at the time of assembly, and the results of every inspection and test. The DHR is not a summary; it is an exhaustive record.
This level of traceability exists for one reason: risk containment. If a field failure occurs or a supplier recalls a specific component lot, the manufacturer must be able to identify every affected unit with speed and certainty. Without complete DHR integrity, a single failed component can force a blanket recall of an entire production run, crippling a product launch because the scope of exposure cannot be determined. Traceability is not administrative overhead; it is the mechanism that protects patients and limits financial damage when something goes wrong.
What a Complete DHR Captures for Every Assembly
A DHR for a PCBA must link every component reel to its supplier certificate of conformance. It must document which equipment was used for each operation and its parameters during the run. It must record which qualified operators performed setup, inspection, and testing. It must capture environmental data like temperature and humidity if the materials are sensitive. It must also log the results of all in-circuit and functional testing, including the serial number and calibration status of the test equipment itself. Finally, it includes any deviation reports if an anomaly occurred and was dispositioned during the build.
A manufacturer must have systems to capture this data at every step. While manual, paper-based DHR systems are permissible, they invite latency and error. A paper traveler accompanying boards on the factory floor can be filled out incorrectly, be misplaced, or have steps missed. If this happens, the DHR is incomplete, and the entire batch is in jeopardy. Here, the quality of a manufacturer’s digital infrastructure directly determines its speed and reliability.
How Real-Time DHR Systems Prevent Retrospective Delays

A mature ISO 13485 operation uses integrated software to capture DHR data in real time, as a natural byproduct of production. When an operator scans a component reel into a pick-and-place machine, the system automatically logs the lot number and timestamp. When a board exits a reflow oven, thermal profile data is linked to the batch. When a functional test runs, results are written directly to the DHR database. The DHR is complete the moment the production run ends.
This architectural difference eliminates the single largest source of lead time inflation: the scramble to assemble documentation after the fact. In facilities with manual systems, compiling records and resolving discrepancies can take days or weeks after the boards are built. The product is finished but cannot ship because the paperwork is not. In a real-time DHR system, production and documentation are synchronized. When the last board is tested, the DHR is ready. This is how discipline enables speed.
UDI Labeling: A Workflow Discipline, Not a Final Step
The Unique Device Identification (UDI) system is a global regulatory framework for tracking medical devices. For PCB assemblies, UDI labeling isn’t just about printing a barcode at the end of the line. It is a workflow discipline that must be integrated into the production process with strict data integrity controls, ensuring each device’s identity is correctly established and linked to its DHR.
A UDI is a regulatory identifier, not an internal serial number. It follows a globally harmonized format (typically GS1 or HIBCC) and includes a Device Identifier (DI) for the product model and a Production Identifier (PI) containing the lot number, serial number, or manufacturing date. Because the format is dictated by regulators, the customer must provide the UDI data and labeling specifications, and the manufacturer must execute them with precision.
When UDI Integration Occurs in the Assembly Process
The point at which a UDI label is applied depends on the device. For some products, the label is applied directly to the PCBA after final testing. For others, the PCBA is a subassembly, and the final UDI label is applied later by the customer. In the former case, the PCBA manufacturer must manage UDI generation, application, and verification as a controlled step in the workflow.
A common mistake is treating UDI labeling as a post-production task handled in the shipping department. This introduces unacceptable risk. If labels are applied manually without being tied to the DHR system, there is no automated check that the correct label was applied to the correct unit. The best practice is to integrate UDI printing and application directly into the production line, with barcode scanning or vision systems to verify that the label content matches the unit’s DHR record.
The Mechanics of Serialization and Label Verification
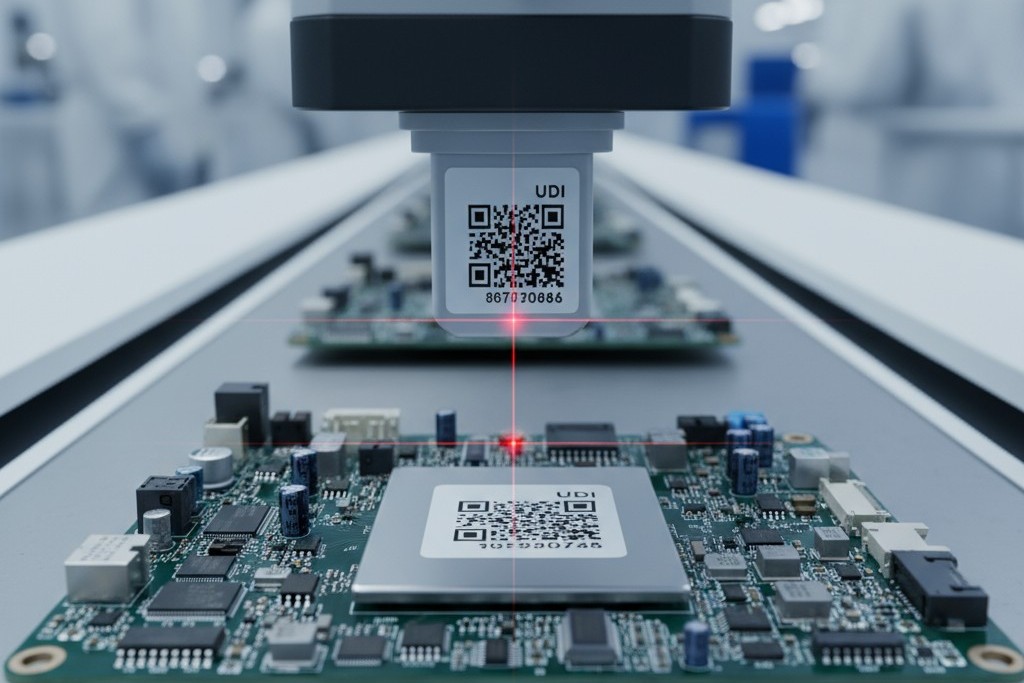
When unit-level serialization is required, the manufacturer must generate a unique serial number for each board and encode it into the UDI label. This requires a serialization system that interfaces with the DHR database to prevent duplicates and permanently associate each serial number with its specific unit history. The verification step, typically an immediate scan after application, confirms the label is readable and correct, automatically flagging any unit that fails. A mature partner will have a defined design transfer process to verify the completeness of all UDI specifications before production begins, preventing costly downstream disruption.
Process Validation: Building in Repeatability
Process validation is the formal, documented proof that a manufacturing process will consistently produce assemblies that meet all requirements. It is not process development; it is the final demonstration that a process, operated within defined parameters, will yield conforming output every time. Under ISO 13485, critical processes must be validated before they are used for routine production.
For PCBA manufacturing, processes like reflow soldering, conformal coating, and automated inspection require validation. The rationale is simple: if you cannot fully verify the quality of an outcome after the fact—like the internal integrity of a solder joint—you must prove the process itself is capable beforehand. When you can’t inspect quality in, you must build it in.
What Process Validation Proves and When It Occurs
A full process validation follows a formal protocol: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that the equipment is installed and documented correctly. OQ confirms the equipment operates properly across its intended range of parameters. PQ is the most rigorous phase: it involves running production-representative assemblies through the process to demonstrate, through testing, that the output consistently meets all acceptance criteria.
This validation must be completed before the process is used for commercial production. While this requires an upfront investment of time for a new product, a validated process enables speed later. It qualifies the process for routine use, allowing production to proceed without extensive per-unit verification.
The Relationship Between Validation and Flexibility
A validated process is not a locked process, but any changes must be managed through a formal change control procedure. A minor adjustment within the validated range may not require action, but a major change—like introducing a new component type or switching solder paste—will trigger a revalidation requirement.
A manufacturer with a mature validation program has clear procedures for evaluating these changes and executing revalidation efficiently. They can draw on historical data to respond quickly when a change is necessary. A manufacturer with weak discipline treats every change as a crisis, requiring lengthy revalidation efforts that delay production. The difference is not the regulation; it is the maturity of the system designed to meet it.
Controlled Storage and Environmental Monitoring
A medical device assembly does not become inert after it leaves the production line. It remains subject to environmental risks that can degrade quality. Controlled storage extends the quality system into the post-production phase, protecting assemblies from electrostatic discharge (ESD), moisture, contamination, and mix-ups until they are delivered.

ISO 13485 requires that storage conditions be defined, monitored, and documented. For PCBAs, this means robust ESD protection, temperature and humidity management for moisture-sensitive components, and cleanliness protocols to prevent particulate contamination. The rigor of these controls scales with the device’s risk profile.
Categories of Control: ESD, Environment, and Contamination
ESD is a well-known risk, but it takes on heightened significance for medical devices where a latent defect may not manifest until clinical use. Storage areas must be ESD-protected, and assemblies must remain in protective packaging until they reach another controlled environment. Temperature and humidity control is critical for moisture-sensitive components or for coatings that require specific curing conditions. These areas must be equipped with systems that continuously log environmental data and trigger alarms for out-of-spec conditions. Contamination control is vital for assemblies used in implantable or sterile devices, requiring dedicated storage areas with controlled access and air filtration.
Traceability in Storage: Lot Segregation and FIFO
Beyond environmental factors, storage is a traceability checkpoint. Assemblies must be stored to prevent mix-ups between lots or product versions. This requires physical segregation, clear labeling, and an inventory system that enforces lot identification. First-in, first-out (FIFO) discipline is standard practice to minimize shelf-life risks. Every storage event—from receipt into the stockroom to shipment—must be recorded and linked to the DHR, creating an unbroken chain of custody that allows for a rapid, precise response to any post-market issues.
Continuous Audit Readiness: The System That Prevents Disruption
An ISO 13485 audit is not a surprise. Surveillance audits from certification bodies and regulatory audits from agencies like the FDA occur on a schedule or in response to a trigger event. The content of these audits is also not a mystery; auditors examine records, observe processes, and verify that documented procedures are being followed.
The difference between a seamless audit and a disruptive one is the state of the operation. When a manufacturer operates in a state of continuous compliance—where every process generates audit-ready records in real time and deviations are dispositioned immediately—the audit becomes a verification, not a discovery. Production doesn’t stop. Engineers aren’t pulled into frantic documentation searches. The auditor’s questions are answered with data that is already compiled and accessible.
How Daily Discipline Creates Perpetual Audit Readiness
Continuous audit readiness means the quality system is always prepared for an unannounced inspection. There is no “special preparation mode.” The records an auditor requests—DHRs, Corrective and Preventive Actions (CAPAs), validation protocols, training matrices, calibration logs—are maintained as part of daily operations, not compiled in a rush when an audit is scheduled.
This state is achieved through automation and discipline. Digital DHR systems eliminate backlogs. Automated calibration tracking flags equipment before it expires. Training management systems ensure only qualified personnel perform tasks. CAPA systems track issues from identification to closure. These are not audit tools; they are production tools that generate audit-ready outputs.
What Auditors Examine and How Systems Respond
When auditors enter a PCBA facility, they request DHRs, validation records, and evidence of change control. They observe the production floor and interview personnel. A system that is continuously compliant responds without delay. DHRs are pulled from the database in minutes. Validation records are in a structured repository. The CAPA database shows that issues are tracked to closure. The auditor’s visit becomes a confirmation, not an investigation.
Contrast this with an operation where compliance is periodic. The audit triggers a scramble. Engineers hunt for old reports. Production is paused to clean up paperwork. The audit itself uncovers discrepancies that internal systems should have caught. The audit becomes a source of delay and costly corrective actions, not because the auditor is adversarial, but because the system was not ready. This is how a manufacturer whose quality system is embedded in daily operations avoids disruption and protects lead times.
Evaluating an ISO 13485 PCBA Partner: The Criteria That Matter
A manufacturer either holds a valid ISO 13485 certificate or they do not. But operational maturity exists on a spectrum, and that spectrum determines whether a partner will accelerate your program or introduce friction. The evaluation should focus on the systems that reveal true discipline.
Certification vs. Maturity: Ask to see the most recent surveillance audit report. An absence of major nonconformances is a good sign. Ask how long the manufacturer has held certification; longevity suggests embedded discipline. Verify the certificate’s scope explicitly includes PCBA contract manufacturing.
System Integration: Request a live demonstration of the DHR system. The ability to pull a complete record for a specific lot in real time is a powerful indicator. Ask how UDI labeling is integrated and verified. Review a process validation report for a similar product; its depth and clarity reveal the program’s maturity.
Transparency in Design Transfer: The design transfer process is where ambiguities create downstream delays. A mature partner will have a formal checklist to verify UDI requirements, critical process parameters, and test criteria before production begins. Ask to see this procedure. Its rigor is predictive of the partnership’s success.
Red Flags: Be wary of a manufacturer that cannot produce DHRs quickly or relies on paper-based systems for critical records. Be wary of a facility where environmental monitoring is manual and infrequent. Be wary of a partner who cannot clearly explain their change control process or who has experienced lapses in certification. These are signs that the quality system exists on paper but not in practice.
The choice of a manufacturing partner affects not only your product’s quality but also your speed to market. A partner whose ISO 13485 system is genuinely embedded in their operations will not slow you down with compliance overhead. They will accelerate your program by eliminating the rework and fire drills that plague those who treat compliance as a checkbox. The discipline enables the speed.