The impulse to track everything is strong. When regulators demand traceability and auditors scrutinize records, the safest-seeming response is to capture every data point, scan every barcode, and map every component to every serial number. This instinct is expensive. It is also, in many cases, a misallocation of resources that creates the illusion of control without delivering proportional risk reduction.
Lot traceability exists for one reason: to enable targeted action during a failure investigation or recall. It is a mechanism for isolating affected units and limiting exposure, not a comprehensive insurance policy against all defects. The distinction is critical because the cost of traceability scales with its depth. Full serial genealogy for every resistor and capacitor on a high-volume disposable device can double data handling overhead and introduce chokepoints at every assembly step. That cost must be justified by a corresponding reduction in actual risk, not by the theoretical comfort of having more data.
The challenge, then, is one of calibration. Too little traceability leaves manufacturers vulnerable to broad, expensive recalls when a single defective lot could have been surgically isolated. Too much traceability stalls throughput, buries quality teams in data noise, and creates audit liabilities when the system inevitably develops gaps under production pressure. The answer is not a universal standard but a risk-based framework that matches traceability depth to the consequences of failure—a system that is defensible, not just exhaustive.
What Lot Traceability Actually Accomplishes
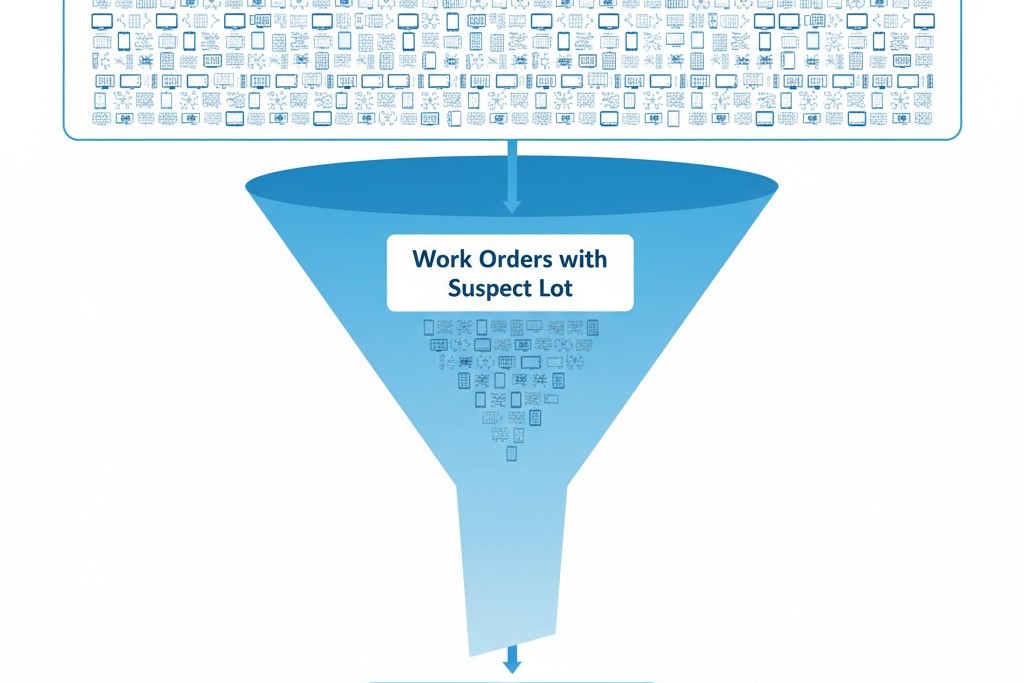
At its core, traceability is recall infrastructure. Its primary function is to answer a single question when a defect is discovered: which finished devices contain the suspect component lot, and where are those devices now? The speed and precision of that answer determine the scope of the recall, the cost of the corrective action, and the manufacturer’s liability. A robust traceability system can narrow a potential recall from tens of thousands of units to hundreds. A weak system forces a broad recall because the data required to isolate the problem simply does not exist or cannot be retrieved quickly.
The mechanism is straightforward. Components arrive with supplier lot codes. Assemblies are built in production runs, often grouped by work orders. Finished devices receive unique serial numbers. Traceability is the linkage between these identifiers. Lot-level traceability connects a component lot to a batch of finished devices, while serial-level traceability connects it to individual devices. The depth of that linkage determines the granularity of any recall.
A common misconception is that more traceability equals more safety. This is false. Safety is a function of design robustness, process controls, and inspection rigor. Traceability does not prevent defects; it enables faster, more targeted responses when defects occur. For example, a component defect affecting one supplier lot, used across 50 work orders of 200 devices each, creates a potential exposure of 10,000 units. If traceability links component lots to work orders, the recall can target only the specific work orders that consumed the defective lot, potentially shrinking the recall to 2,000 units. If traceability only exists at an annual level, all 10,000 units are at risk. The law of diminishing returns applies: each additional layer of traceability delivers less incremental precision while adding linear or exponential data handling costs.
The Three Depths of Component Traceability
Traceability is not a binary choice. There are three operationally distinct depths, each defined by the granularity of the component-to-device linkage. The choice between them determines system complexity, throughput impact, and the practical scope of recall actions.
Lot-Level Tracking for Purchased Assemblies
The baseline approach is lot-level tracking, which records which supplier lot codes were received and during which time period they were consumed in production. The linkage is temporal and probabilistic, not deterministic. If a defective lot is identified, the manufacturer can infer that devices built during the consumption window potentially contain those components. The recall scope is broad but bounded.
Data requirements are minimal. Receiving logs capture incoming lot codes, and production records note the date range or work orders during which components were issued. No scanning occurs at the assembly step itself; the linkage is established retrospectively by cross-referencing consumption and build records. This approach is sufficient when the cost of a broad recall is acceptable. It is common for commodity components in low-risk devices—resistors, capacitors, and standard fasteners in a high-volume disposable diagnostic. A defect is unlikely to cause patient harm, the component cost is negligible, and the manufacturer can afford to recall all devices built during a multi-week window without catastrophic financial impact.
Component-to-Device Mapping at the Work Order Level

A more pragmatic middle ground links specific component lot codes to specific production batches, typically defined by work orders. The linkage here is deterministic at the batch level: the system records which component lots were consumed by which work orders, and which device serial number ranges correspond to those work orders. If a defect is found, the recall targets only the devices built from the affected work order.
Data requirements are moderate. Barcode scanning or manual logging occurs when components are issued to a work order, and the Manufacturing Execution System (MES) or batch record captures the lot-to-work-order linkage. The result is a two-hop traceability chain: component lot to work order, work order to serial number range. This is the default for most medical device manufacturers balancing compliance and efficiency. It provides surgical recall capability without requiring one-to-one component tracking. The throughput impact is manageable because scanning occurs at kitting or issuance, not every assembly operation. The risk reduction is substantial: a recall that would affect 10,000 units might shrink to just 500 under this model.
Full Serial Genealogy for Every Component
The most exhaustive approach is full serial genealogy, which records which specific component serial number or lot code was installed in which specific device. The linkage is one-to-one for every traceable component. If a defect is identified, the system can generate a list of exact device serial numbers containing the defective part, enabling unit-level recalls or patient notifications.
The data requirement is immense. Barcode scanning occurs at every assembly step where a traceable component is installed, with each scan linked to the device serial number in real time. For a device with 50 traceable components and a production volume of 100,000 units per year, the system must capture and store five million records annually. This depth is justified only when the consequences of failure are severe and patient-specific action is required. Implantable devices are the canonical example. A defective pacemaker lead or spinal implant must be traceable to the individual patient because the corrective action is surgical revision. The cost of the system is trivial relative to the liability and the ethical imperative of patient safety.
Where Traceability Depth Genuinely Reduces Risk
The decision framework for choosing a traceability depth should be based on risk, not just compliance. Regulations specify that traceability must exist, but they rarely prescribe the depth. The manufacturer must justify its choice by assessing failure consequences, recall costs, and patient harm potential. The goal is to match traceability depth to the granularity required for a proportional and effective corrective action.
Implantable and life-sustaining devices demand full serial genealogy for critical components. A cardiac pacemaker contains a battery, a pulse generator, leads, and a hermetic housing. A defect in any of these can cause device failure and patient death. The only appropriate response is patient-specific notification, which requires one-to-one traceability from the component to the device to the patient’s medical record. Here, the traceability system is life-safety infrastructure, and its cost is non-negotiable.
High-volume disposables with short service lives and low individual risk need only lot-level or work order-level traceability. For a disposable blood glucose test strip or single-use surgical instrument, a component defect may cause a malfunction, but the patient harm is limited. The corrective action is a product replacement, not a surgical intervention. The appropriate recall granularity is at the production batch level: identify the affected lots, notify distributors, and remove the product from the supply chain. Serial genealogy adds no meaningful risk reduction because the manufacturer cannot and does not need to identify which patient used which specific test strip.
Active, non-implantable devices like diagnostic imaging systems or infusion pumps occupy the middle ground. A component defect can cause device failure and indirect patient harm, but the failure is observable, and the corrective action is repair or replacement. Traceability requirements depend on the component’s failure mode. Power supplies and sensors affecting device accuracy require work order-level or serial-level traceability. Structural components or user interface elements may only require lot-level tracking. The distinction comes from failure consequence analysis: if a defect can cause undetected measurement error or unsafe operation, tighter traceability is justified.
Barcode and MES Integration Without Throughput Collapse
A traceability system’s operational impact is determined by where and how data is captured. Poorly designed systems introduce friction at every step. Well-designed systems automate capture at critical control points, minimize manual intervention, and degrade gracefully when components lack machine-readable codes.
Automated Capture at Critical Control Points

The most effective systems integrate automated barcode scanning into the natural production flow. The principle is to capture data when an operator is already handling the part, not to insert a dedicated scanning step. The highest-value capture points are component kitting and final assembly verification. A scan during kitting establishes the lot-to-work-order linkage for dozens of components at once. A scan during final inspection can confirm the device serial number and prompt for scans of critical components if serial genealogy is required. This approach achieves the necessary depth with minimal disruption.
The data architecture must support fast writes and indexed queries. Each scan generates a database transaction, and for high-volume production, the system must handle thousands of scans per hour without stalling the line. Cloud-based MES platforms offer elastic scaling, but on-premise systems remain common where data sovereignty and validation are paramount. For components without supplier lot codes, like custom machined parts, manufacturers must generate internal lot identifiers at receiving. This is an acceptable trade-off for non-critical parts, though it means traceability terminates at the receiving dock.
Manual Systems for Low-Volume or Legacy Lines

Not all environments justify the cost of full automation. Low-volume lines and prototype builds often rely on manual systems using paper batch records or spreadsheets. Operators record component lot codes by hand or affix barcode labels to the batch record as parts are issued and installed. At the end of the run, the record is scanned or transcribed to create a permanent traceability file.
This approach is disciplined but fragile. Transcribing records is slow and error-prone, making data unavailable for immediate queries. A mock recall can take hours or days of manually searching through records, a significant audit risk. Regulators accept manual systems for low-volume production but scrutinize them for error rates and slow retrieval times. A common transition strategy is to automate incrementally, starting with kitting and serialization. This hybrid approach uses automated scans to create a traceability backbone and manual entries to fill the gaps, balancing cost and capability.
What Auditors Actually Verify
Auditors evaluate traceability systems on two fronts: data integrity and retrieval capability. Integrity means the records are complete, accurate, and tamper-evident. Capability means the system can identify affected devices quickly enough to support a real-world recall. The audit doesn’t require maximum depth, but it demands that the chosen depth is consistently maintained and demonstrably functional.
The core audit activity is the mock recall. An auditor selects a component lot code and asks the manufacturer to identify all finished devices containing that lot. The system must produce this list within hours, not days. This is a stress test of the architecture, data quality, and operational readiness. A system that requires manual searching of paper records or runs queries that time out under load will fail.
Common failure modes are predictable. Incomplete linkage occurs when operators skip scans under pressure. Unreadable barcodes force manual entry, spiking error rates. But the most serious failure is missing records—lost batch records or inaccessible database archives. Regulators see this not as a traceability gap but as a systemic failure of the quality system.
Ultimately, the audit is binary. Either the manufacturer can demonstrate complete traceability for the tested lot, or it cannot. Partial traceability is a failure because the gap represents uncontrolled risk. The cost of failure is not just a regulatory finding, but production delays and reputational damage from demonstrating inadequate control over a core quality function.