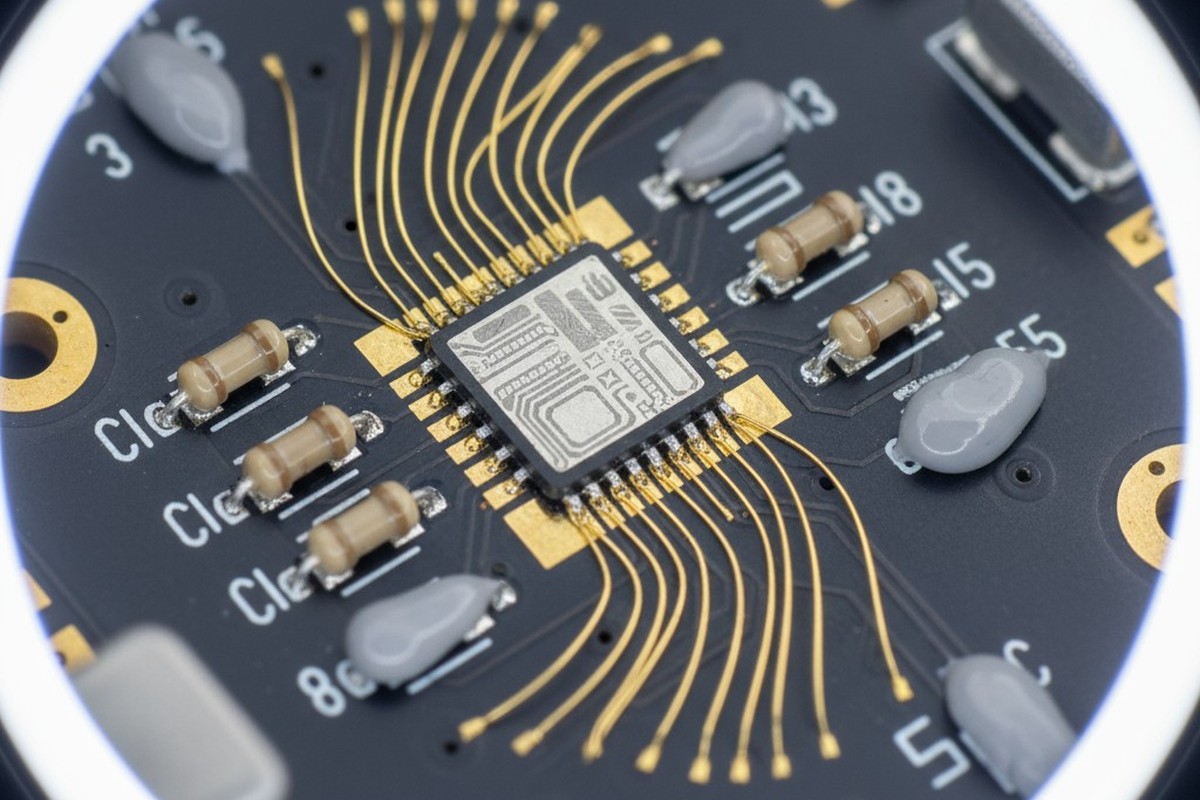
Designs that combine gold wire bonding with surface-mount technology occupy an uncomfortable middle ground in PCB fabrication. Wire bonding demands a pure, soft noble metal surface for reliable thermosonic or ultrasonic connections. Soldering requires a surface that promotes wetting and the formation of intermetallic compounds with tin-based alloys. These requirements are not complementary. In most material systems, they are fundamentally opposed.
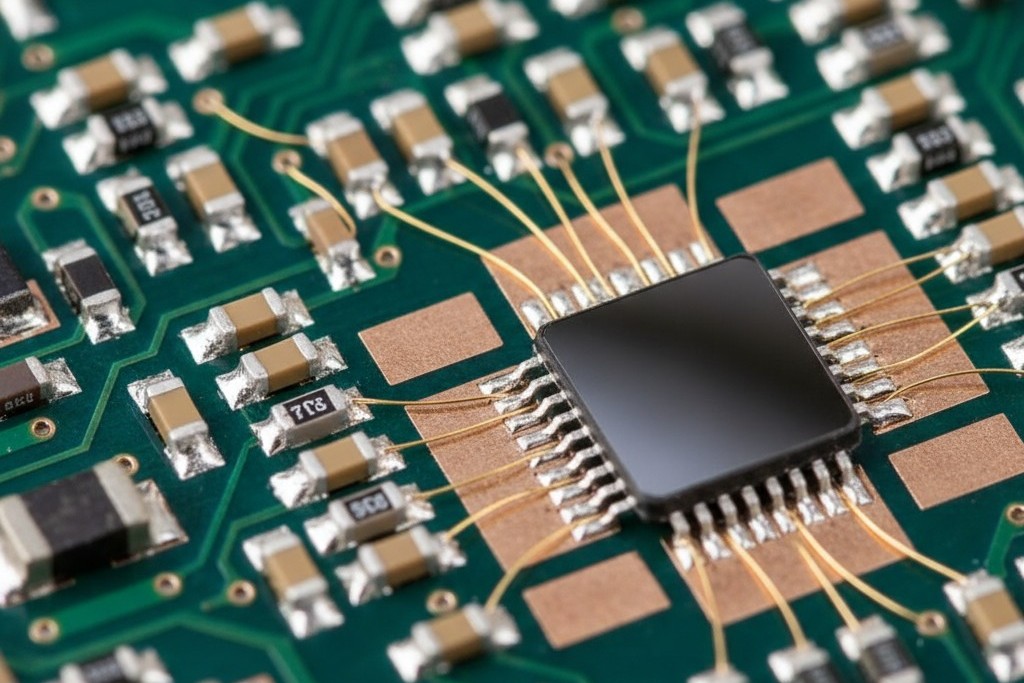
For years, engineers threaded this needle with compromises: thick gold over nickel for some pads, different finishes for different areas, or simply accepting degraded performance in one process to enable the other. Each workaround introduced complexity, cost, or reliability risk. ENEPIG, or Electroless Nickel Electroless Palladium Immersion Gold, eliminates the compromise by satisfying both processes on a single surface finish. It achieves this through a specific material stack that leverages the distinct properties of each layer.
This is not a simple choice. ENEPIG introduces its own challenges, most notably the risk of “black pad” during plating and lingering questions about nickel corrosion. At Bester PCBA, we have seen both the failures that result from poor process control and the exceptional reliability that comes from doing it right. The finish works, but only when the plating process and assembly parameters are managed with absolute precision. This is the case for ENEPIG in mixed assemblies—how it works, and what it takes to avoid its failure modes.
The Surface Finish Conflict in Mixed-Technology Assemblies
Wire bonding is a process of creating a metallurgical connection between a thin gold or aluminum wire and a bonding pad using heat, pressure, and ultrasonic energy. The bond forms through a combination of mechanical deformation and atomic interdiffusion at the interface. For this to happen reliably, the pad surface must be chemically pure, free of oxides, and soft enough to deform under pressure without cracking. Gold is the ideal surface. It does not oxidize, it is soft and ductile, and it allows consistent energy transfer during ultrasonic bonding. The process is well understood and essential for RF modules, power semiconductors, and hybrid assemblies where die must be connected to the substrate.
Soldering operates on an entirely different principle. A solder joint is not an adhesive connection; it is a metallurgical bond formed by creating intermetallic compounds at the interface between the solder and the pad. When molten tin-based solder contacts a copper pad, tin and copper atoms diffuse into one another, forming layers of Cu₆Sn₅ and Cu₃Sn intermetallics. These layers are the bond. The wetting action—the spreading of molten solder across the pad—is governed by the surface energy of the pad finish and the flux’s ability to reduce oxides. A solderable surface must allow rapid intermetallic formation, resist oxidation until it reaches the reflow oven, and avoid forming brittle phases that would compromise the joint.
The conflict arises because gold, while perfect for bonding, is a liability for soldering when its thickness exceeds about 0.5 micrometers. Excessive gold dissolves into the solder joint during reflow and can form a brittle gold-tin intermetallic, AuSn₄. This embrittlement weakens the joint and invites crack propagation under thermal or mechanical stress. Conversely, surfaces optimized for soldering like immersion silver, immersion tin, or organic solderability preservatives are too hard, too prone to tarnishing, or too chemically unstable to support reliable wire bonding.
A designer working on a mixed assembly needs a finish that allows gold wire to bond with low resistance and high pull strength while also allowing solder paste to form robust joints. Standard single-layer finishes cannot do both. ENEPIG can.
How ENEPIG Resolves Incompatible Requirements
ENEPIG is a multi-layer surface finish consisting of three distinct metallic layers deposited sequentially onto the copper pad: electroless nickel, electroless palladium, and immersion gold. Each layer serves a specific function, and the finish’s performance depends on maintaining precise control over the thickness and composition of all three.
The Layer Structure and Material Properties

The foundation is a layer of electroless nickel, typically 3 to 6 micrometers thick, that serves as a diffusion barrier. It stops copper from migrating to the surface and oxidizing. This nickel isn’t pure; it’s an alloy containing 6 to 9 percent phosphorus by weight, deposited through an autocatalytic chemical reduction. That phosphorus content is non-negotiable. Too little, and the nickel becomes susceptible to the corrosive attack that causes black pad. Too much, and it becomes brittle, compromising the mechanical integrity of the solder joint.
Next is the key to ENEPIG’s dual functionality: a thin layer of palladium, usually 0.05 to 0.15 micrometers. Although thin, its role is outsized. As a noble metal, palladium resists oxidation and tarnishing, forming reliable Pd₂Sn and PdSn intermetallics with tin-based solders for a strong metallurgical bond. During reflow, this palladium layer dissolves into the solder joint, becoming part of the intermetallic structure. Crucially, it also protects the underlying nickel from oxidation, giving the finish a much longer shelf life than bare nickel or nickel-gold systems.
The final surface is an ultra-thin flash of immersion gold, typically just 0.03 to 0.08 micrometers. Its primary job is to protect the palladium from oxidation and contamination during storage and handling. This gold layer is thin enough that it dissolves rapidly and harmlessly into the solder during reflow, allowing the joint to form primarily with the palladium. For wire bonding, however, this whisper-thin gold provides the pure, soft interface required for ultrasonic energy to form a strong metallurgical bond between the wire and the pad.
Why Palladium Enables Dual Compatibility
Palladium is the linchpin. It resolves the contradictory demands of soldering and wire bonding.
For soldering, it acts as a perfectly wettable surface. It doesn’t readily oxidize, so the flux can focus on removing minor contaminants instead of a heavy oxide layer. The intermetallic compounds it forms with tin are stable and mechanically sound. Because the palladium layer is thin and dissolves into the joint, it avoids the embrittlement issues associated with the thicker gold used in other finishes.
For wire bonding, the palladium layer is essentially transparent. The bond forms on the immersion gold surface, and the ultrasonic energy passes through the thin gold and palladium without interference. The palladium does not inhibit the bond; in fact, its relative hardness can even improve pull strength by providing a more stable subsurface. The result is a single finish where both the solder joint and the wire bond achieve full performance, with no compromise.
Why Common Alternatives Fail the Mixed-Assembly Test
Understanding why ENEPIG is necessary requires looking at why more common surface finishes are inadequate for these demanding applications. Each alternative fails to satisfy one of the two core requirements.
ENIG and the Bondability Problem
For many years, Electroless Nickel Immersion Gold (ENIG) was the default finish for high-reliability applications. It uses the same electroless nickel barrier as ENEPIG but is capped with a thicker layer of immersion gold, often 0.05 to 0.15 micrometers or more. While this surface is excellent for wire bonding, it creates a serious problem for soldering.
The thicker gold layer dissolves into the solder joint during reflow. If the gold concentration becomes too high, it forms brittle AuSn₄ intermetallics. These hard compounds are prone to cracking under thermal cycling or mechanical stress, leading to a solder joint with a shorter fatigue life and a higher risk of field failure. While some designers try to control ENIG gold thickness to stay below the embrittlement threshold, this introduces process variability and risk. Furthermore, ENIG carries the same black pad risk as ENEPIG without offering any advantage in soldering performance. For a mixed assembly, it simply trades one problem for another.
Immersion Silver and Tin: Unsuitable for Wire Bonding
Immersion silver (ImAg) and immersion tin (ImSn) are common lead-free finishes optimized for soldering. ImAg provides good wettability and forms strong Cu-Sn intermetallics directly at the copper interface. ImSn is a cost-effective alternative that also forms reliable solder joints.
Neither is suitable for wire bonding. Silver tarnishes in the presence of sulfur, common in many industrial environments, and this tarnish layer prevents the intimate metal-to-metal contact required for a bond. Immersion tin is harder than gold and forms a native oxide layer that interferes with the bonding process. Worse, tin is prone to whisker formation—thin, crystalline filaments that can grow and cause electrical shorts, a non-starter for high-reliability applications.
Organic solderability preservative (OSP) coatings, which are thin layers of organic flux, offer no bonding surface at all. Each of these single-layer finishes optimizes for one process at the expense of the other. ENEPIG was engineered to eliminate this trade-off.
Black Pad: Risk and Prevention
The most significant risk with ENEPIG is black pad, a failure mode where weak or non-existent adhesion between the nickel and gold layers leads to solder joint failure. The name comes from the black, discolored appearance of the nickel surface after the gold is pulled away. This is not a theoretical problem; it has caused catastrophic field failures and remains the primary process control challenge for any ENEPIG plater.
The Mechanism of Failure

Black pad occurs during the immersion gold plating step. This is a galvanic displacement process: the board’s nickel surface is immersed in a gold salt solution, where gold ions deposit onto the nickel while nickel atoms are oxidized and dissolve into the solution. This exchange is normal.
The problem begins when the nickel corrodes excessively. If the nickel has a high phosphorus content (above 10-11%) or the gold plating bath is too aggressive from excessive temperature, high gold concentration, or low pH, the nickel surface can corrode faster than the gold deposits. This leaves behind a layer of nickel oxide or phosphide at the interface. This layer has poor adhesion. When solder is applied, it wets the gold and palladium but cannot bond to the corroded nickel beneath. The joint looks acceptable but has virtually no mechanical strength and can fail with minimal stress.
Non-Negotiable Process Controls
Preventing black pad is a matter of strict process control. Three variables are critical: the nickel’s phosphorus content, the gold bath chemistry, and the palladium layer quality.
First, the nickel phosphorus content must be maintained between 6 and 9 percent. Below this range, the nickel is less uniform; above it, the nickel becomes more reactive and vulnerable in the gold bath. Plating shops must continuously monitor and control their nickel bath chemistry, including concentrations of nickel ions, reducing agents, and stabilizers.
Second, the immersion gold bath must be operated to minimize nickel attack. This means controlling the pH (4.5 to 5.5), keeping the gold ion concentration low, and maintaining the bath temperature below 70°C. Modern gold bath formulations include corrosion inhibitors specifically to protect the nickel, and their use is essential.
Third, the palladium layer must be dense and uniform. It acts as a protective barrier, reducing the nickel’s exposure to the gold bath. If the palladium is porous or incomplete, the gold bath can penetrate and cause localized corrosion. Finally, since ENEPIG uses a very thin gold layer, the immersion time is short, which inherently reduces the opportunity for nickel attack compared to thicker ENIG finishes.
These controls are not optional. A plating shop that cannot demonstrate consistent control over these variables should not be fabricating ENEPIG boards. At Bester PCBA, we require evidence of process capability from our suppliers, including microsection analysis and adhesion test data. Black pad is preventable, but prevention requires discipline.
Nickel Corrosion: A Manageable Concern
A secondary concern with ENEPIG is the potential for in-service galvanic corrosion between the nickel and gold layers. Because gold is significantly more noble than nickel, theory suggests that in the presence of an electrolyte, the nickel could corrode if exposed. This has led some to hesitate in adopting ENEPIG for harsh environments.
While not unfounded, field evidence suggests this concern is overstated in well-manufactured assemblies. The palladium layer is the critical protective element. It isolates the nickel from direct contact with the gold, mitigating the galvanic couple. During soldering, the palladium dissolves into the joint, and the nickel remains sealed beneath a stable intermetallic structure, unexposed to the environment.
Long-term reliability studies of ENEPIG in automotive, telecom, and industrial applications show failure rates comparable to or better than other high-performance finishes. Failures attributed to nickel corrosion are rare and are almost always traced back to design flaws—such as exposed nickel at board edges due to poor solder mask coverage or contamination from flux residues—rather than the finish itself.
Standard design practices can further mitigate this already low risk. Conformal coating provides a moisture barrier, and proper solder mask design ensures nickel is not exposed. When process controls are maintained and basic design rules are followed, ENEPIG provides robust, long-term reliability.
Ensuring Reliable Soldering with ENEPIG
While designed for dual compatibility, ENEPIG’s soldering performance still depends on a well-controlled assembly process. The finish is forgiving, but optimization ensures consistent, high-yield results.
Solder Paste and Flux Chemistry
ENEPIG is compatible with standard tin-silver-copper (SAC) lead-free solder alloys like SAC305. The resulting intermetallic phases, primarily Pd₂Sn and PdSn, are stable and provide excellent mechanical strength and thermal cycling performance.
Because ENEPIG surfaces are highly resistant to oxidation, an aggressive flux is not required. A no-clean flux with moderate activity (ROL1 or similar) is generally sufficient. More aggressive fluxes can be used, but they may require post-reflow cleaning to remove corrosive residues.
Reflow Profile and Shelf Life
Standard lead-free reflow profiles work well with ENEPIG, with peak temperatures of 240-250°C and a time above liquidus of 60-90 seconds. During reflow, the thin gold and palladium layers dissolve completely into the solder, and the joint forms primarily at the nickel interface. Because the total gold thickness is so low, the risk of gold embrittlement that plagues ENIG is eliminated.
The shelf life of ENEPIG-finished boards is excellent. The gold and palladium layers protect the underlying nickel from oxidation, allowing for storage of 12 months or more in controlled environments without any degradation in solderability. This is a significant advantage over immersion silver or tin, which tarnish more readily.
For designs that require both wire bonding and SMT soldering, ENEPIG is not just a viable option. It is the only mainstream finish that delivers full performance in both processes without forcing a compromise.