What is Bond Strength
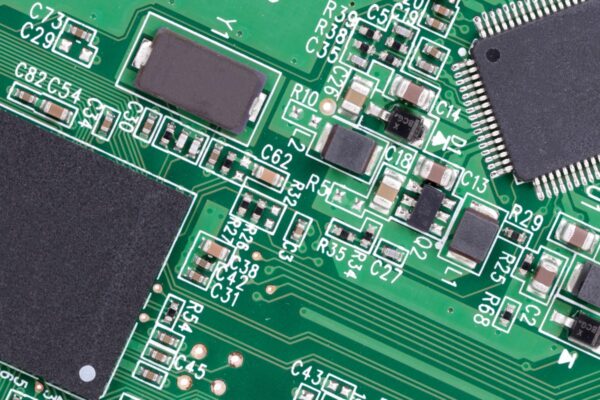
Bond strength is the force per unit area required to separate two adjacent layers of a PCB by a force perpendicular to the board surface. It is a measure of the strength of the bond between different layers of the PCB.
The bond strength determines the reliability and durability of the PCB assembly. It ensures that the bonded materials can withstand external forces and stresses without breaking or separating. The strength of the bond can vary depending on various factors, including the materials being bonded, the method of bonding, and the quality of the solder mask or coating used.
The bond strength can be influenced by the adhesion properties of the materials involved in the bonding process. For example, when the assembly is directly bonded to the substrate or metal, the bond strength can be quite high. Additionally, the bond strength is not limited by the size or area of the bonded components, allowing for flexibility in the design and construction of PCB assemblies.
Furthermore, the bond strength maintains a secure and tight seal. This prevents water ingress or leakage and allows for effective cooling systems in the PCB assembly. The ability of the bond to withstand temperature variations is also important, ensuring that the bonded assembly remains stable and reliable across a wide temperature range.
Frequently Asked Questions
How Do You Measure Bond Strength
The measurement of bond strength is determined by the bond dissociation energy, which refers to the energy needed to break a specific covalent bond in a mole of molecules. It is important to note that multiple bonds are generally stronger compared to single bonds involving the same atoms.
What Is Bond Strength Testing
Bond strength testing typically involves evaluating the amount of force needed to break a bond created by an adhesive between two metal blocks. This test often includes assessing the shear and flexural bond strength of a bonding agent or comparing different bonding agents in different environmental conditions.
What Does High Bond Strength Mean
A high bond strength refers to the strength of a bond, indicating that the molecule containing that bond is stable and less likely to react. In contrast, compounds with lower bond energies tend to be more reactive. The table provided in Table 9.12 lists various bond energies in kJ/mol.
Does Higher Bond Strength Mean More Stable
The bond with the highest bond energy is considered the most stable. A stable system is characterized by low energy levels. Therefore, a bond is formed when the electron energy level is at its lowest.